Electron Emission
Electron Emission: Overview
This topic consists of various concepts like Photoelectric Emission,Work Function of a Metal,Photoelectron, etc.
Important Questions on Electron Emission
The graph between frequency of incident radiations and stopping potential for a given photosensitive material is as follows.
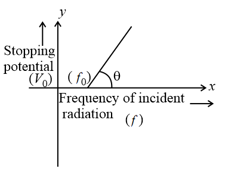
What information can be obtained from the value of the intercept on the potential axis?
A metal surface when exposed to ultraviolet radiations for a short interval of time was found to acquire positive charge. What happened to the metal surface?
Given below are two statements:
Statement: Photovoltaic devices can convert optical radiation into electricity.
Statement: Zener diode is designed to operate under reverse bias in breakdown region.
In the light of the above statements, choose the most appropriate answer from the options given below:
The difference between threshold wavelengths for two metal surfaces and having work function and in is:
{Given, }
If radiation of energy is incident on the material with work function of then,
If radiation of energy is incident on the material with work function of then,
The light emitted in the transition to (where is the principal quantum number of the state) in hydrogen is called -light. Find the maximum work function that a metal can have so that -light can emit photoelectrons from it.
The metal which has the highest work function in the following is
If the threshold wavelength for photoelectric effect on sodium metal is then find its work function.
Which of those metal having least work function among them?
Will electrons eject when an incident ray of is strike on a metal plate of threshold frequency .
In another experiment, a source of constant intensity and variable frequency is incident on a metallic surface. The graph shows the variation of the stopping potential with photon frequency , for a particular value of intensity.
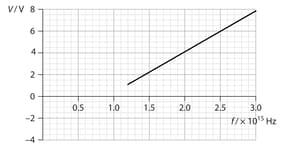
Use the graph to estimate the Planck constant obtained from this experiment.
In another experiment, a source of constant intensity and variable frequency is incident on a metallic surface. The graph shows the variation of the stopping potential with photon frequency , for a particular value of intensity.

Use the graph to estimate the work function of the metallic surface.
What are photoelectrons in physics?
What does thermionic emission means? List the factors affecting thermionic emission.
The time lag between a photon hitting the surface of a metal and the emission of a photoelectron is
The work function of Platinum is twice that of Calcium. If the minimum photon energy required to emit photoelectrons from the surface of Platinum is then for Calcium, the minimum photon energy would be
When green light is incident on a certain metal surface, electrons are emitted but no electrons are emitted by yellow light. If red light is incident on the same metal surface
What are photoelectron ?
What do you mean by thermionic emission?
